It is an important topic and has its presence in both PHARMACEUTICAL ANALYSIS and PHARMACEUTICAL INORGANIC CHEMISTRTY.
FROM THE NAME ONLY we can understand that it is something related to the ‘formation of precipitate.’
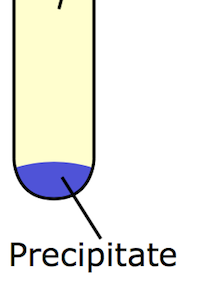
Here we can see that the precipitate are the solid chunks of particles present at the bottom of the solution.
As we study about Precipitation titrations we come across the word GRAVIMETRY !
Remember that :
- Precipitation titration is the part of Gravimetry.
In Gravimetric titrations too we study the formation of precipitate i.e., the solid insoluble particles that rest at the bottom of the solution, separated from the supernatant solution.
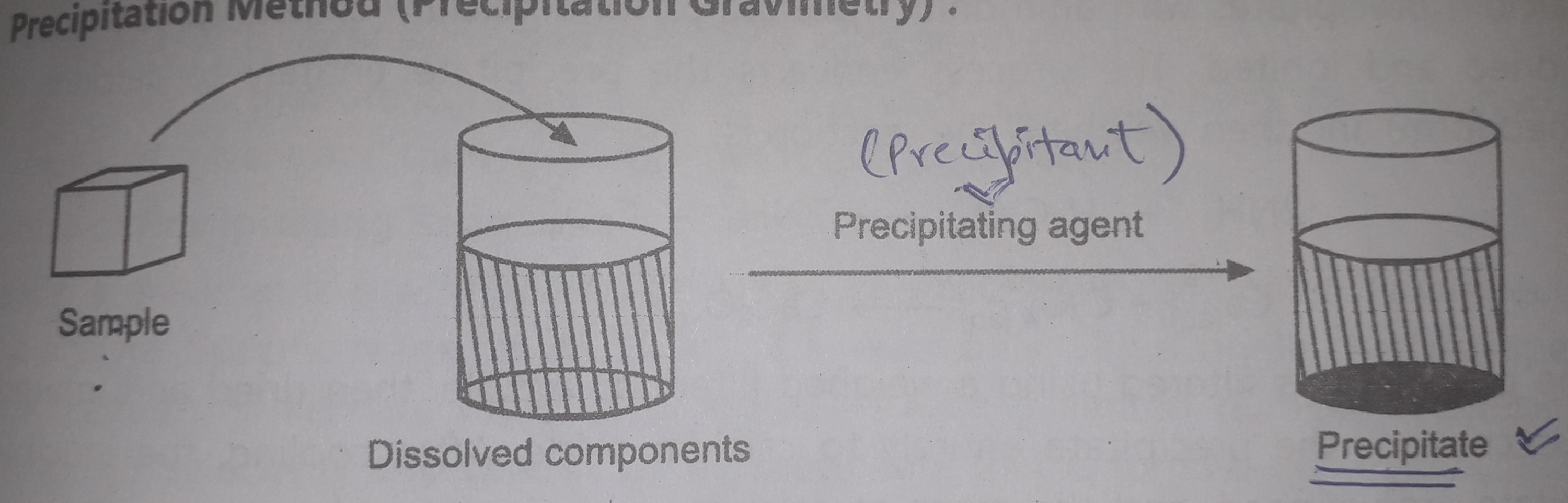
You can see the formation of precipitatr with the help of a precipitating agent.
Precipitation titration has been divided into following methods :
- Mohr’s Method
- Fajan’s Method
- Volhard’s Method
- Modified Volhard’s Method
Before explaining them further I would like you to tell that MOHR and FAJAN method are ARGENTOMETRIC TITRATIONS.
Now, what is Argentometric titration?
Argentometric titrations can be defined as following ;
- These titrations help in the determination of Chloride present in the sample.
- Titration is done by AgNO3.
- These titrations involve silver(I) ion .
- Formation of AgCl as a white precipitate occurs.
Now keeping this information in mind we can now understand better the methods explained below.
1. MOHR’S METHOD

- Mohr’s Method is an Argentometric titration.
- It means that it contains AgNO3 in the burette i.e., it will titrate the solution or sample present in the conical flask below.
- The conical flask contains NaCl. K2CrO4 ( Potassium Chromate ) is an indicator.
- The first reaction is of AgNO3 with NaCl which forms the AgCl precipitate which is a white ppt. (Primary ppt.)
- The second reaction is of AgNO3 with K2CrO4 to form Ag2CrO4 (silver chromate) which is a reddish brown colour ppt. ( secondary ppt. )
Reaction :
AgNO3 + NaCl —–>AgCl (ppt.) + NaNO3
Next Reaction :
AgNO3 + K2CrO4 —> Ag2CrO4 + KNO3
NOTE : Analysis of Iodine cannot be done by this because its primary ppt will also be reddish brown in colour. Thus we will not be able to distinguish between primary and secondary ppt. !!
2. FAJAN’S TITRATION

- Argentometric titration.
- It is based on surface adsorption. Adsorption is the sticking or addition of particles on the surface of adsorbent.
- Being an example of Argentometric titration , it contains AgNO3 in the burette and NaCl is present in the conical flask present below the burette.
- Fluorescein is the indicator used here i.e. , a negatively charged indicator.
- First the reaction of AgNO3 occurs with NaCl to give AgCl as a white ppt.
REACTION :
AgNO3 + NaCl —> AgCl + NaNO3.
- Then at the end, Fluorescein adsorps on the white ppt. and gives a pink colour ppt.
NOTE : This titration has to be performed in a DARK PLACE only ! OTHERWISE , in LIGHT the fluorescein layer will not be formed, thus the colour will not turn pink and we will not get the END POINT !!!
In both Mohr’s and Fajan’s method the pH is NEUTRAL.
3. VOLHARD’S METHOD

- In this method the burette contains Ammonium Thiocyanate (NH4SCN).
- In the conical flask below we have our sample i.e., AgNO3.
- The indicator used is Ferric Alum (Iron-3-Ammonium Sulphate)
- Also HNO3 is added in the conical flask so as to have an ACIDIC pH.
- The reaction of NH4SCN takes place with AgNO3 to form AgSCN which is the white ppt formed here.
REACTION :
AgNO3 + NH4SCN —> AgSCN+NH4NO3
- Then the reaction of NH4SCN occurs with ferric alum to form Fe(SCN)3 which is a reddish yellow coloured ppt.
REACTION :
NH4SCN + NH4Fe(SO4)2 —-> Fe(SCN)3
NOTE: It is a DIRECT TITRATION !
4. MODIFIED VOLHARD’S METHOD

In this method too the burette contains NH4SCN and the conical flask below contains AgNO3 (in excess) .
The indicator present is Ferric alum.
Excess of AgNo3 is present because it is a Back Titration.
Back titration ,is a titration method where the concentration of an analyte is determined by reacting it with a known amount of excess reagent.
The remaining excess reagent is then titrated with another, i.e., the second reagent. The second titration’s result shows how much of the excess reagent was used in the first titration, thus allowing the original analyte’s concentration to be calculated.
Now,
The excess of AgNO3 reacts with NaCl which is our sample to form AgCl which is a white precipitate.
Reaction :
1.) AgNO3 + NaCl —> AgCl + NaNO3 + unreacted AgNO3
Now,
2.) NH4SCN + AgNO3 —> AgSCN + NH4NO3
3)NH4SCN+ferric alum indicator–> Fe(SCN)3
REMEMBER :
- With the help of unreacted AgNO3 we will determine the value of Cl.
- Masking of AgCl has to be done as it can react with NH4SCN or any other compound.
So we will add ;
Nitrobenzene or dibutyl phthalate. As it will form a layer on AgCl and it will thus not react with NH4SCN.