Be yourself; Everyone else is already taken.
— Oscar Wilde.

This is the first post on my new blog. I’m just getting this new blog going, so stay tuned for more. Subscribe below to get notified when I post new updates.
Keep feeding your mind with knowledge.
Be yourself; Everyone else is already taken.
— Oscar Wilde.

This is the first post on my new blog. I’m just getting this new blog going, so stay tuned for more. Subscribe below to get notified when I post new updates.
Complexometric titrations are used for the determination of metal ions.
These occurs where Covalent Bonds are formed i.e., sharing of electrons occur. These metals will not ionize like in Acid-Base Titrations.

Diagram explains the presence of :
EDTA or Ethylenediaminetetraaceticacid in the burette.
EDTA is a ;
1.) Complexing agent
2.) Chelate i.e., a compound containing a ligand (typically organic) bonded to a central metal atom at two or more points.
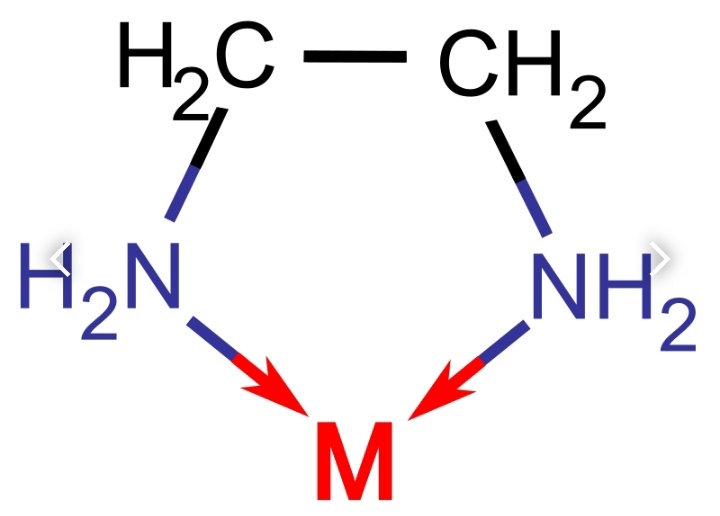
Here the EDTA is joined with a Metal (M) ion by 2 coordinate bonds.
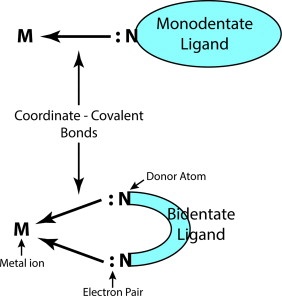
3.) Ligand
4.) Sequestering agent is a dyeing auxiliaries which is used during dyeing for removing hardness of water.Sequestering agents combine with calcium and magnesium ions and other heavy metal ions in hard water. They form molecules in which the ions are held so securely (sequestered) that they can no longer react.
COMPLEXOMETRIC TITRATION TAKES PLACE AS FOLLOWS :
It is an important topic and has its presence in both PHARMACEUTICAL ANALYSIS and PHARMACEUTICAL INORGANIC CHEMISTRTY.
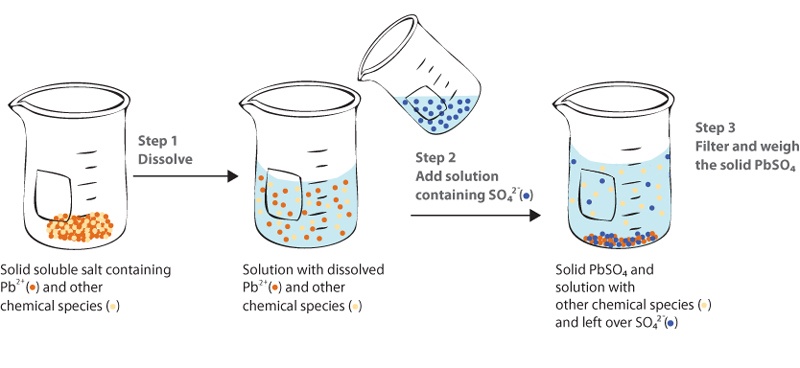
FROM THE NAME ONLY we can understand that it is something related to the ‘formation of precipitate.’
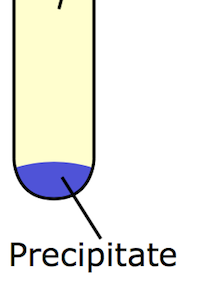
Here we can see that the precipitate are the solid chunks of particles present at the bottom of the solution.
As we study about Precipitation titrations we come across the word GRAVIMETRY !
Remember that :
In Gravimetric titrations too we study the formation of precipitate i.e., the solid insoluble particles that rest at the bottom of the solution, separated from the supernatant solution.
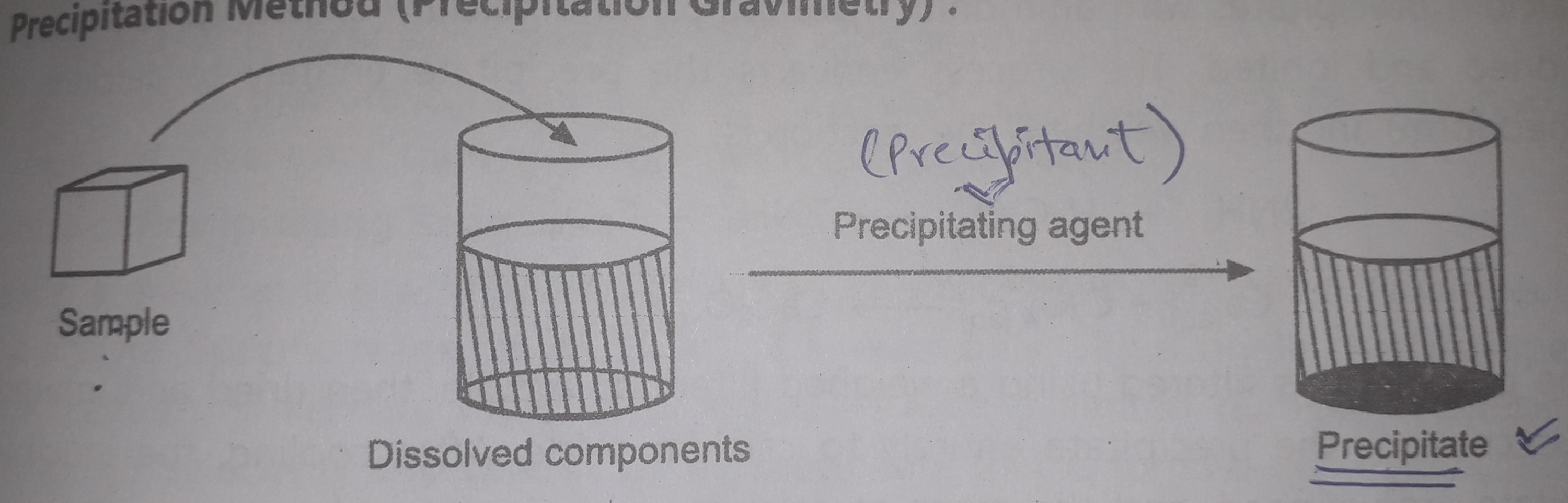
You can see the formation of precipitatr with the help of a precipitating agent.
Precipitation titration has been divided into following methods :
Before explaining them further I would like you to tell that MOHR and FAJAN method are ARGENTOMETRIC TITRATIONS.
Now, what is Argentometric titration?
Argentometric titrations can be defined as following ;
Now keeping this information in mind we can now understand better the methods explained below.
1. MOHR’S METHOD

Reaction :
AgNO3 + NaCl —–>AgCl (ppt.) + NaNO3
Next Reaction :
AgNO3 + K2CrO4 —> Ag2CrO4 + KNO3
NOTE : Analysis of Iodine cannot be done by this because its primary ppt will also be reddish brown in colour. Thus we will not be able to distinguish between primary and secondary ppt. !!
2. FAJAN’S TITRATION

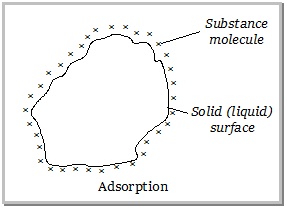
REACTION :
AgNO3 + NaCl —> AgCl + NaNO3.
NOTE : This titration has to be performed in a DARK PLACE only ! OTHERWISE , in LIGHT the fluorescein layer will not be formed, thus the colour will not turn pink and we will not get the END POINT !!!
In both Mohr’s and Fajan’s method the pH is NEUTRAL.
3. VOLHARD’S METHOD

REACTION :
AgNO3 + NH4SCN —> AgSCN+NH4NO3
REACTION :
NH4SCN + NH4Fe(SO4)2 —-> Fe(SCN)3
NOTE: It is a DIRECT TITRATION !
4. MODIFIED VOLHARD’S METHOD

In this method too the burette contains NH4SCN and the conical flask below contains AgNO3 (in excess) .
The indicator present is Ferric alum.
Excess of AgNo3 is present because it is a Back Titration.
Back titration ,is a titration method where the concentration of an analyte is determined by reacting it with a known amount of excess reagent.
The remaining excess reagent is then titrated with another, i.e., the second reagent. The second titration’s result shows how much of the excess reagent was used in the first titration, thus allowing the original analyte’s concentration to be calculated.
Now,
The excess of AgNO3 reacts with NaCl which is our sample to form AgCl which is a white precipitate.
Reaction :
1.) AgNO3 + NaCl —> AgCl + NaNO3 + unreacted AgNO3
Now,
2.) NH4SCN + AgNO3 —> AgSCN + NH4NO3
3)NH4SCN+ferric alum indicator–> Fe(SCN)3
REMEMBER :
So we will add ;
Nitrobenzene or dibutyl phthalate. As it will form a layer on AgCl and it will thus not react with NH4SCN.
Every morning as we get up we must be cheerful , positive and enthusiastic for the day ahead so as to savour each moment of our life.
I came across this amazing quote today !

Starting this blog is a beginning of the flow of the river.
“Everyday is a new oppurtunity to learn .”
This is a quote that goes very well with my blog.
Hope you all have a great day ahead.
Please do :
Here is the syllabus of B.pharmacy beginning with all the subjects that one needs to study while doing B.pharmacy.
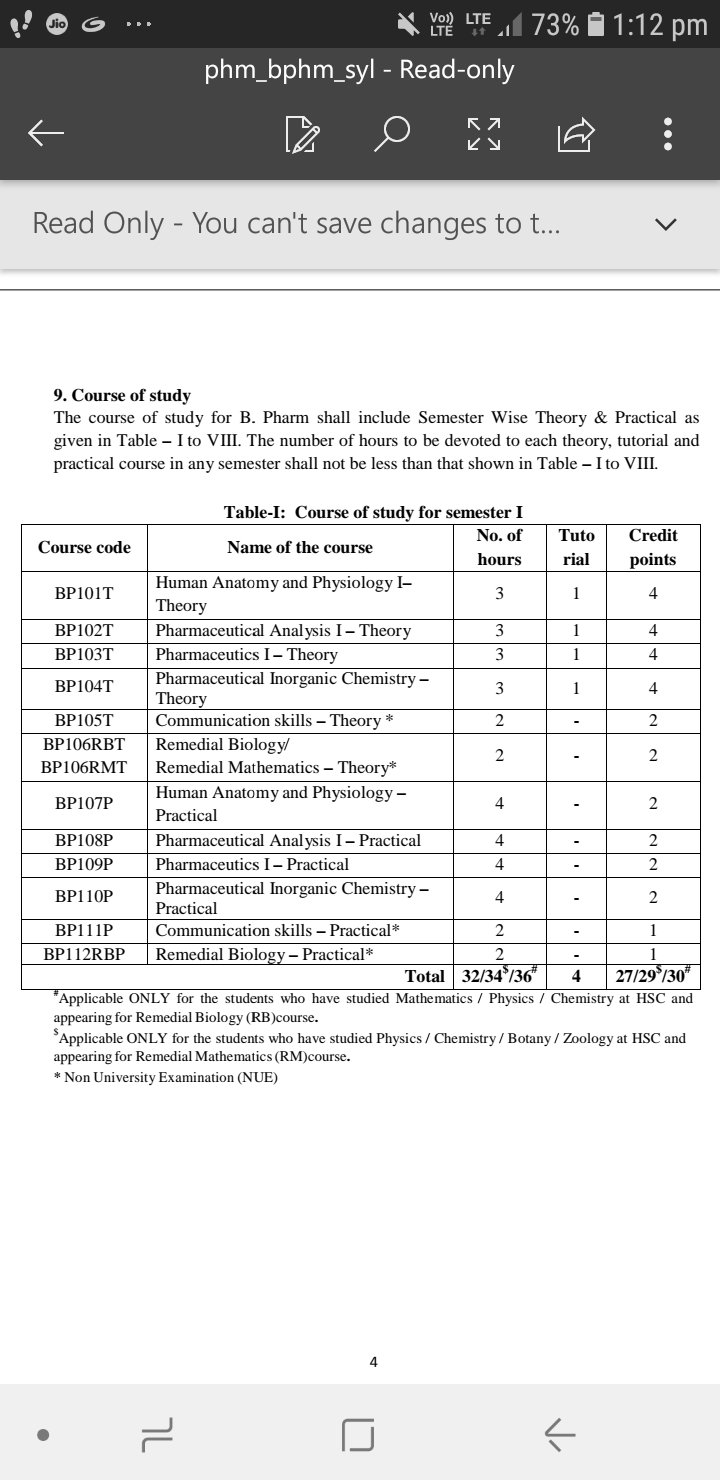
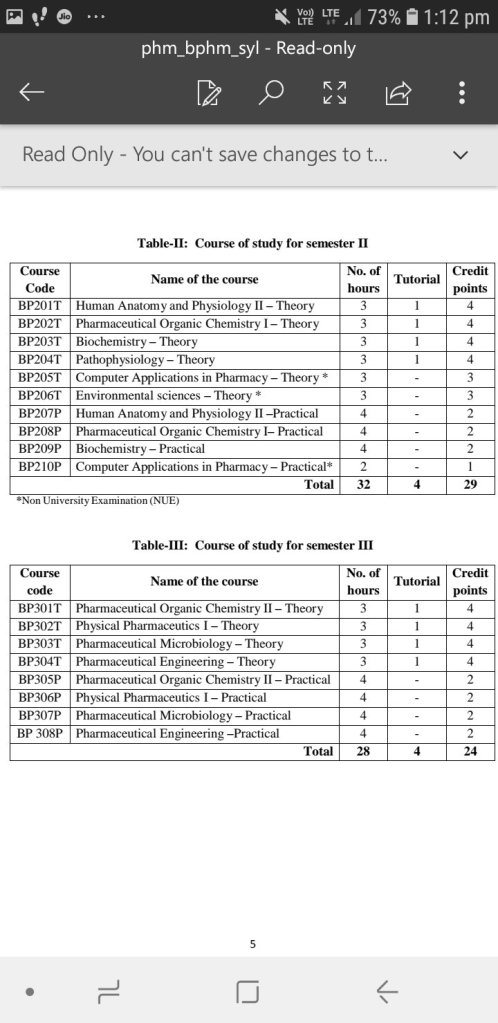
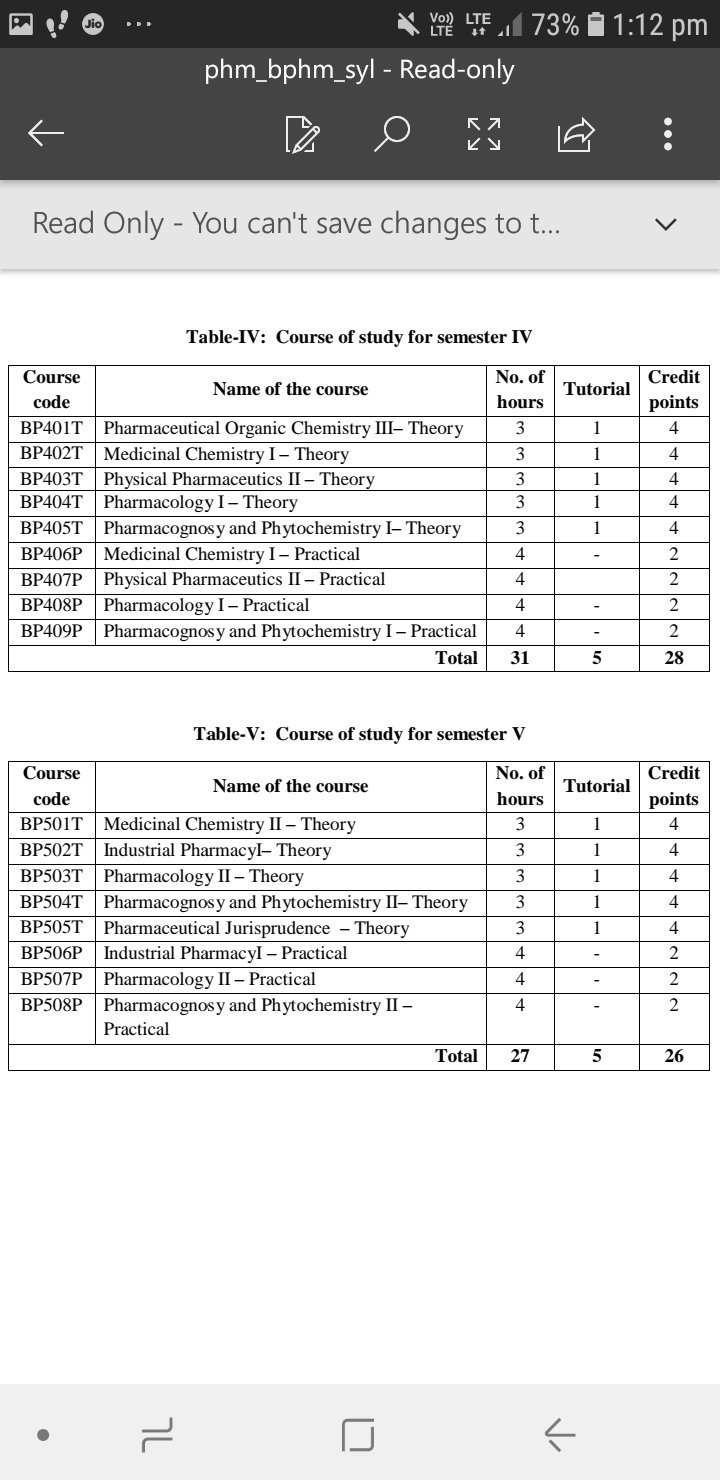
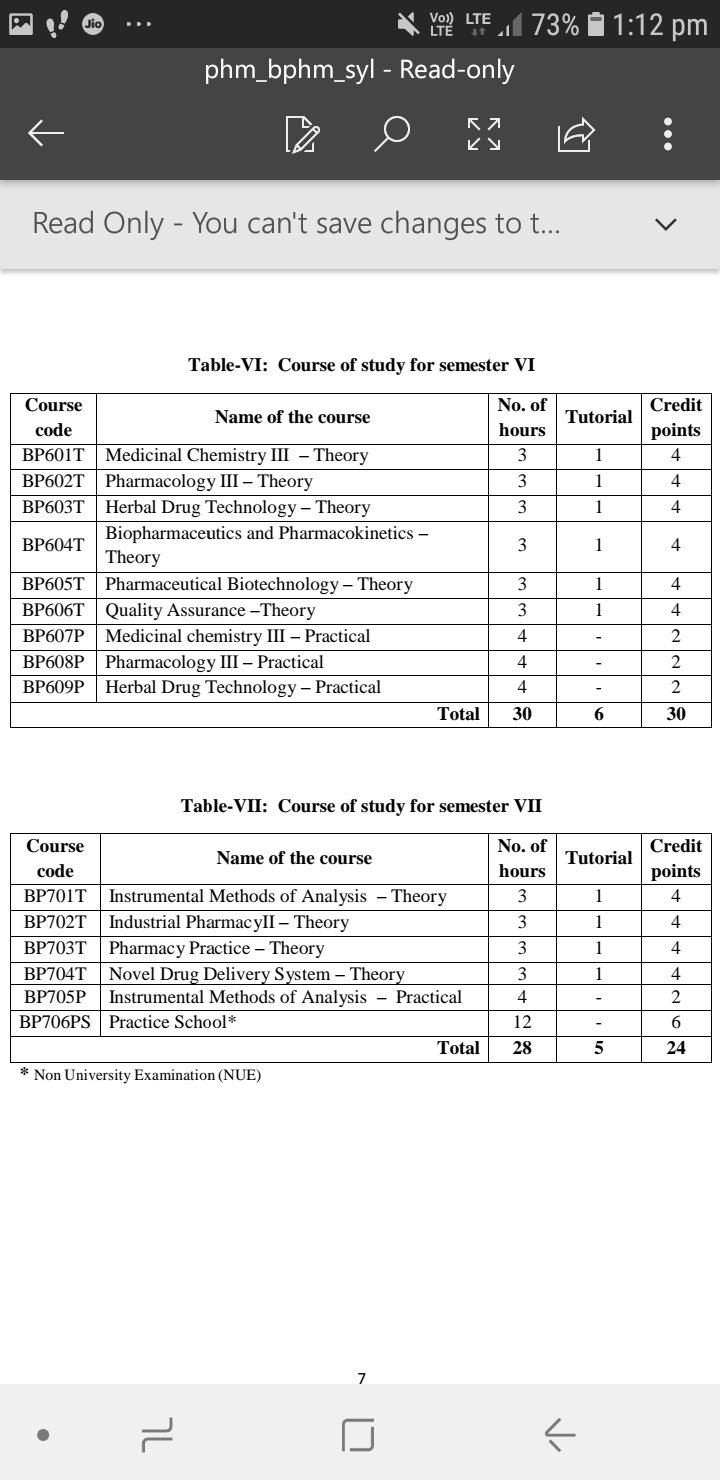
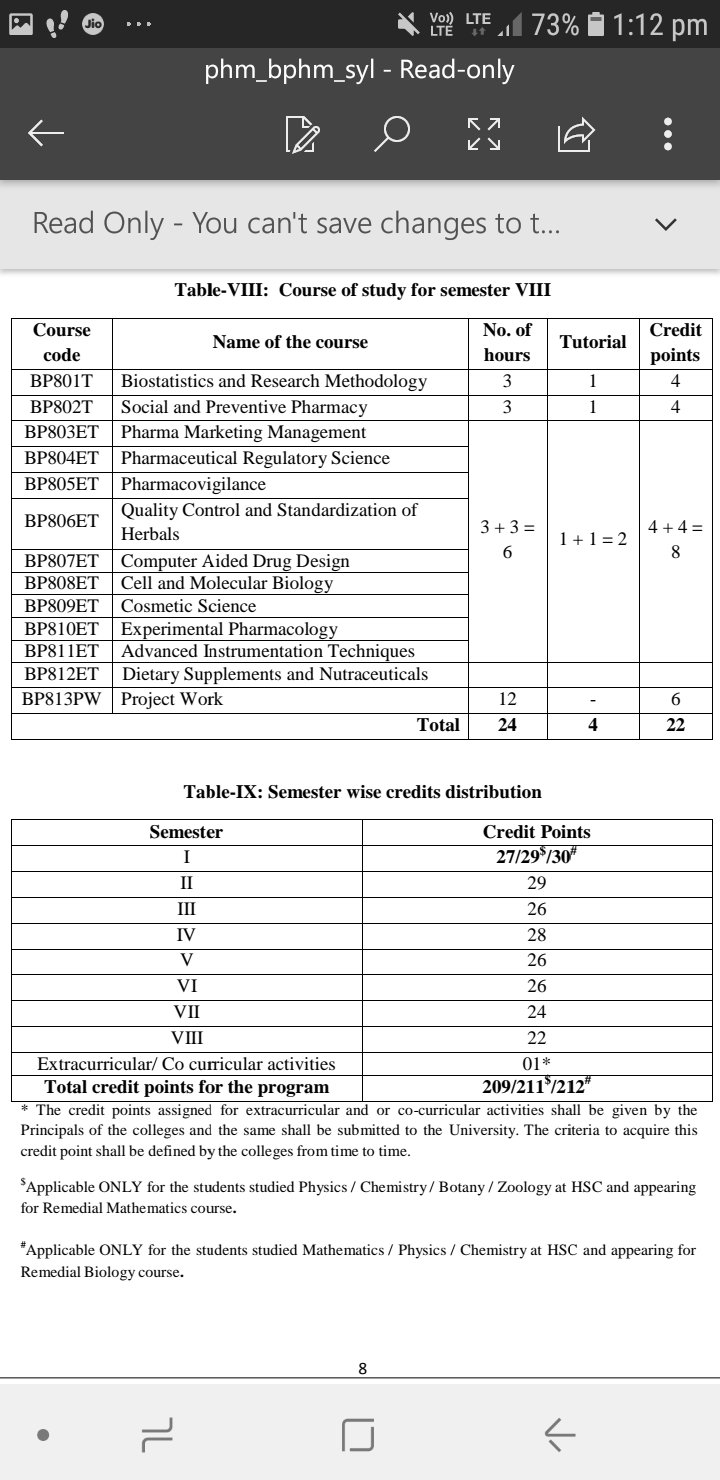
At the end I would like to elaborate the Semester 1 syllabus.
So here it goes !
Human Anatomy and Physiology


Pharmaceutical Analysis


Pharmaceutics


Pharmaceutical Inorganic Chemistry


Communication Skills


Remedial Biology
Students who took PCM have to take Remedial biology in B pharmacy.


Remedial Maths
Students who took PCB have to take Remedial Maths in B. pharmacy.


Students who took PCMB are not required to take these non-university papers i.e., remedial maths and biology.
I hope this will be helpful to you all.
NOTE : This is the information about B pharmacy course. Things may alter according to an individual’s nationality .
Following are the previous year question papers of pharmaceutical inorganic chm. :





Hope you all find this helpful. I will try to find more question papers and will also cover other topics and their explanations will be uploaded soon.
●EDIT : Here are the all previous year question papers. I found the other question papers too now.
Do follow, like and comment.
Following are the previous year question papers of HAP :




I will upload more soon.
●EDIT : Here are the all previous year question papers. I found the other question papers too now.
Hope you all find this helpful.
Following are the question papers of Pharmaceutics :



I will find more question papers and upload it soon. Actually I had many other previous year question papers but I unfortunately deleted it as I had no plan at that time to start a blog .
●EDIT : Here are the all previous year question papers. I found the other question papers too now.
Hope you will like this.
Following are the question papers of the 1st semester subject of B.Pharmacy i.e., Pharmaceutical Analysis :




Hope this will be helpful. The pics were sent to me in the same quality so sorry for the clearity. But you all will surely understand this.
If not then please do tell me.
My name is Aniket Shad. I am a B.Pharma student. I am right now in 2nd semester. I have completed my 12th standard from Springfield Public School. I have got 90.6% in CBSE board, 2018. I have a strong hold on topics related to Biology, Chemistry and right now as I am studying Pharmacy I have good hold on the subjects related to pharmacy too.
I have started to write this blog to spread the knowledge about various topics of :
I am myself a student and I am always willing to improve myself with time. YOU all can comment back for giving me any feedback or topics that I must include in my future blogs.
I am blogging publicly so as to use my free time in a productive manner and if possible to get some financial help from this blog because I have some goals to accomplish.
I WILL TRY TO DILIGENTLY AND PERSISTENTLY write blogs. I hope I will be able to help few students.